Bioavailability Studies to Improve Risk Assessment at SRS
Lynne Fahey McGrathKeith Cooper
Kristie Ellickson
Blake Hart
Paul Lioy
Consortium for Risk Evaluation with Stakeholder Participation and Westinghouse Savannah River Site
This research supported in part by CRESP through the Department of Energy Cooperative Agreement #DE-FC01-95EW55084. This support does not constitute endorsement of views expressed.
What is Bioavailability?
Bioavailability: Is the degree of ability to be absorbed and ready to interact in organism metabolism. (U.S.EPA)
Previous studies have confirmed that bioavailability is a major factor in toxicity of environmental samples (Umbreit et al., 1988). The soil matrix is an important factor in determining a contaminant’s bioavailability. CRESP is developing methods to use bioavailability information to improve risk assessment at DOE sites.
Bioavailability Definition
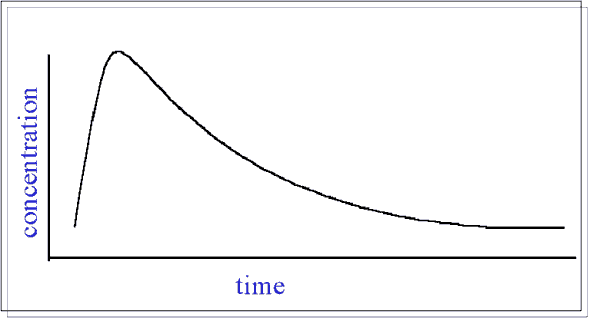
Bioavailability = Rate and Extent of Absorption
Why Study Bioavailability
Bioavailability data can:
- provide realistic information on potential health effects of contamination
- modify defaults using site specific data
- change proposed clean-up levels saving time and money to reach acceptable levels
- help prioritize sites for subsequent evaluation
Methods to Estimate Bioavailability
- Site specific analysis can provide important information that can impact a risk assessment
- In soil, a contaminant’s bioavailability can be estimated in several ways:
- Extraction using acids and bases
- Extraction using biological fluids
- In vitro assays using bacterial systems
- In vivo assays using aquatic organisms or mammals
- Statistical estimation methods
Savannah River Site
- L-Area Rubble Pile (LRP) was a surface depository of general trash. The unit consists of multiple piles of rubble. Visible contents includes scrap metal, construction debris, railroad ties, electrical wiring, and unlabeled cans and bottles.
- LRP was sampled in a preliminary site assessment and found to contain metals, PAHs and PCBs.
- CRESP took parallel samples of the seven definitive locations to examine bioavailability and bioaccessibility of metals located at LBRP.
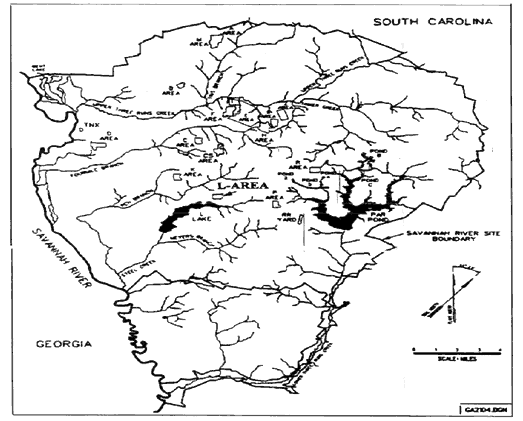
Savannah River Site, South Carolina
CRESP STUDIES
- Hypothesis: bioavailability of soil contaminants will impact risk assessment?
- Site specific analysis can provide important information that can impact decisions in a risk assessment such as:
- Selection of chemicals of concern
- Recommended level of risk
- Level of risk
- Remedial solution
- Regulatory decisions
- Can have broader implications in identifying most efficient and cost effective method for bioavailability assessment
- Can provide information to identify and prioritize sites most impacted by bioavailability data
Study Design: Four assays performed on site samples
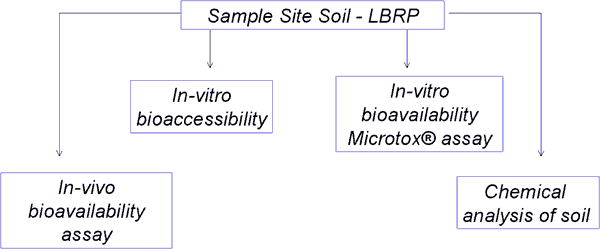
In Vitro Procedures
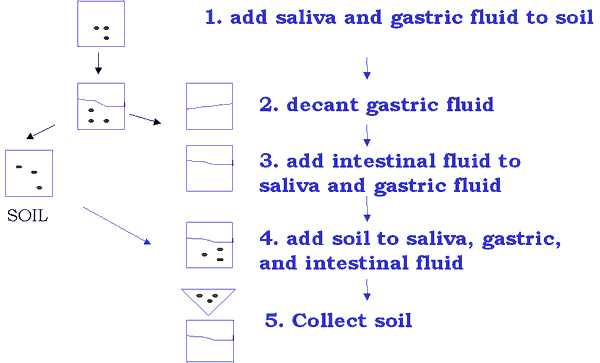
Gastric -vs Intestinal Solubility
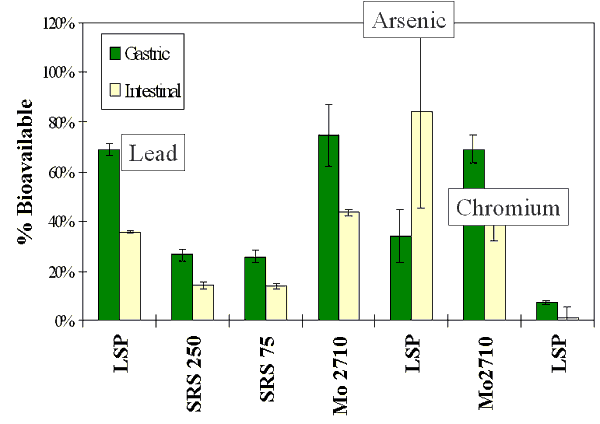
In Vitro Bioaccessibility
- EPA standard methods use high concentrations of harsh acids and bases to extract metals from soil (Fed Reg, 1995)
- This approach generally overestimates bioavailability
- CRESP and EOHSI have developed a two step procedure to extract metals using gastric fluids and saliva
In Vitro Bioaccessibility Data Points
- Solubility of metals within gastric fluid
- Solubility of metals in intestinal fluid, where absorption is likely to occur
- Total mass balance for recovery verification
- Availability of metals from contaminated soil using biological fluids
Soil Analysis Data Points
- Total amount of metals in soil
- Maximum availability of metals
- Baseline for determining percent bioavailable using gastric extraction
- Provides basis for comparison
Importance of Bioaccessibilty Data
- More realistic estimate of bioavailability than chemical extraction
- Less laborious and less expensive than in vivo animal bioavailability studies
- Previous studies have demonstrated that this is a conservative estimate that shows consistency with known bioavailability of metals in soils.
In vitro Bioavailability Procedures
Microtox® Solid-Phase Test puts the test organism (Vibrio fischeri) in contact with contaminated soils. Toxicity of soil is evaluated by examining the inhibition of growth of these organisms.- Soils are diluted
- Flourescence of organisms is measured
- Light output is related to toxicity and bioavailability
Bioavailability Data
- The Microtox® Solid-Phase Test evaluates bioavailability by relating the concentration that comes off a soil particle in aqueous solution to toxicity
- This can be used to screen sites to determine which risk assessments may be impacted by more thorough bioavailability evaluation
In-vivo Bioavailability Data Points
- The Japanese medaka embryo larval assay (ELA) is designed to examine the toxicity of soil.
- Toxic endpoints include lesion occurrence, embryonic development and survival.
- Soils can be tested directly, or eluted at various pH’s or using common solvents
Chemical Analysis of Soil
- Samples were filtered and acidified in nitric acid.
- Samples were analyzed for heavy metals using a Graphite Furnace Atomic Absorption Spectrometer
Importance of Bioavailability Data
- This test can provide information on the impact of pH and solvent extraction on toxicity
- This provides information on the concentration of contaminants likely to be bioavailable.
- This can be used to identify sites that have both toxic concentrations of contaminants which are modified by bioavailability data.
Risk Assessment Using Bioavailability Data
- Information: Comparison of these four methods will provide a range of information regarding bioavailability of metals at LBRP
- Method Evaluation: Will enable determination of efficient and cost effective screen for site bioavailability
- Decision Points: Integration of bioavailability will allow assessment decisions to be compared with and without this information
Metal Availability
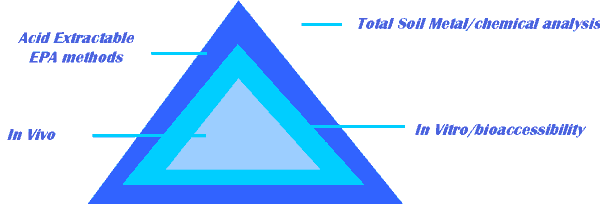 Progression of information with different methods
Progression of information with different methods